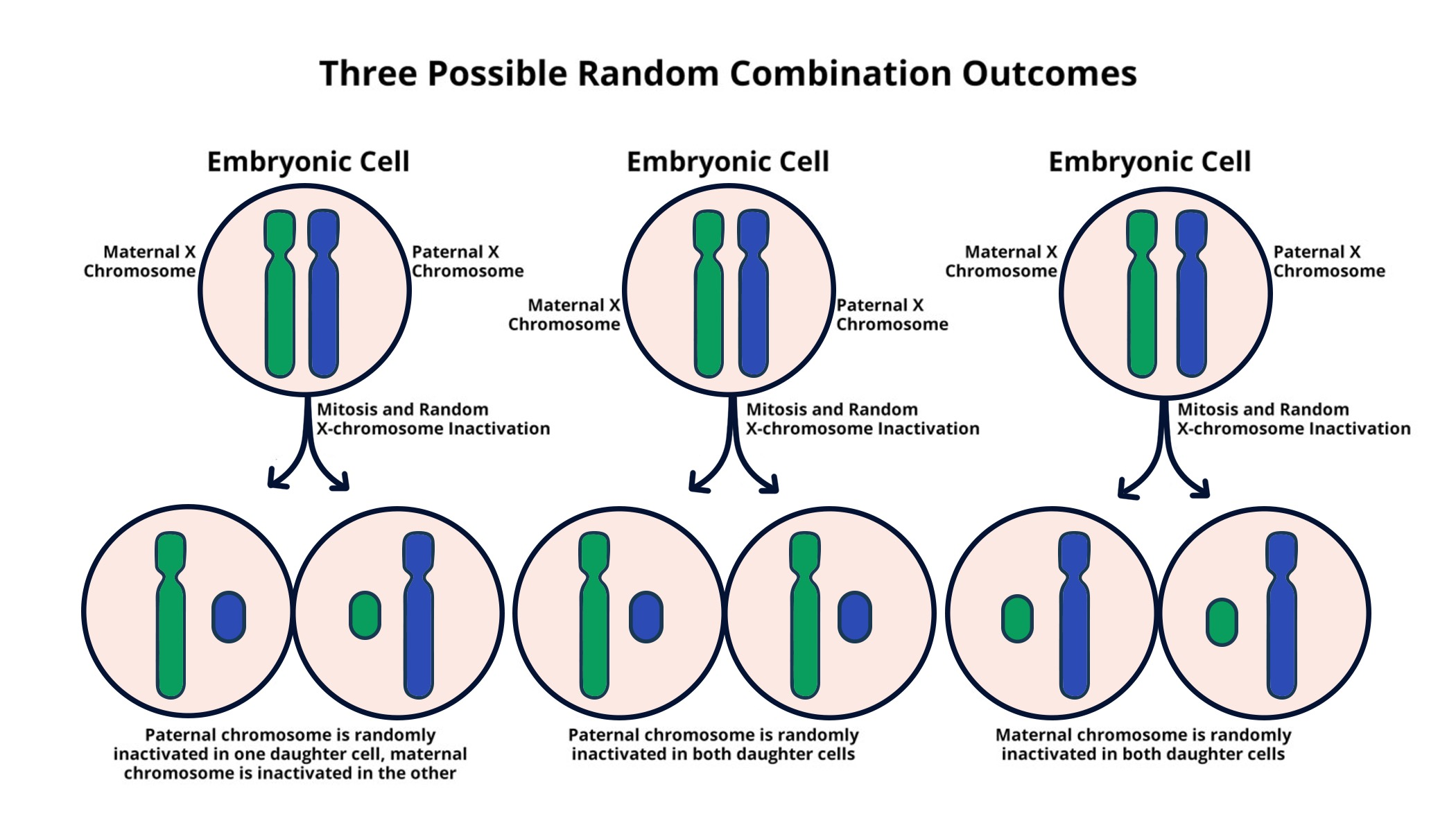
X chromosome inactivation is a fascinating biological process that occurs in females, where one of the two X chromosomes is silenced to balance gene dosage between sexes. This process is key to understanding genetic disorders related to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s groundbreaking research at Harvard Medical School sheds light on the mechanisms behind this chromosomal silencing, opening doors for innovative treatment options. By unraveling the roles of RNA molecules like Xist, researchers have made significant strides toward developing X-linked gene therapies that could potentially restore function to mutated genes. As studies continue to explore these therapies, the work of Jeannie Lee and her team promises new hope for individuals affected by these challenging genetic conditions, bringing us closer to opportunities for improved care and treatment.
The phenomenon of X chromosome silencing is crucial in females, as it ensures the proper functioning of genes linked to the X chromosome. This intricately regulated process, known as X chromosome inactivation, has implications for a range of genetic disorders, including those seen in Fragile X and Rett Syndromes. Recent investigations led by researchers like Jeannie Lee highlight advances in understanding how gene expression is modulated on the X chromosome, paving the way for new therapeutic strategies. Gene therapy targeting X-linked disorders is gaining traction as studies illustrate how the inactivation mechanism can be utilized to reactivate healthy genes. By redefining our approach to X-linked genetic conditions, we may soon see transformative treatments that significantly enhance the quality of life for those affected.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a fundamental biological process that occurs in female mammals, ensuring that gene dosage between sexes is balanced. In females, two X chromosomes exist, but only one is active at any given time, while the other becomes largely inactive through a complex mechanism. This silencing process is crucial because it prevents the potential overexpression of X-linked genes, which could lead to developmental issues and diseases. Researchers like Jeannie T. Lee have made significant strides in unraveling the mysteries of XCI, demonstrating how specific genes and their products, such as Xist RNA, contribute to this intricate process. Their findings highlight the gelatinous structure surrounding chromosomes, which plays a critical role in maintaining proper gene function and regulation during X inactivation.
Moreover, understanding XCI’s mechanisms is vital for the potential development of therapies for genetic disorders linked to the X chromosome. By targeting and manipulating the XCI process, scientists aspire to reactivate silent genes that may harbor healthy versions of mutated genes, such as those linked to Fragile X Syndrome or Rett Syndrome. These insights not only deepen our comprehension of X-linked gene regulation but also pave the path towards innovative therapeutic strategies that could address a myriad of genetic disorders.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetics?
X chromosome inactivation (XCI) is a biological process that occurs in females, where one of the two X chromosomes is randomly silenced to prevent an excess of X-linked gene expression. This mechanism is crucial in maintaining gene dosage balance between males (who have one X) and females. Understanding X chromosome inactivation is essential for developing therapies for genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
How does Jeannie Lee’s research impact the treatment of X-linked genetic disorders?
Jeannie Lee’s research focuses on the mechanisms of X chromosome inactivation and has revealed promising approaches to potentially reactivate silenced genes on the X chromosome. Her findings could lead to innovative therapies for genetic disorders, including Fragile X Syndrome and Rett Syndrome, by uncovering ways to ‘unsilence’ mutated genes that could restore their function.
What role does the RNA molecule Xist play in X chromosome inactivation?
The RNA molecule Xist is key in the X chromosome inactivation process. It is produced from one of the X chromosomes and attaches to that chromosome, altering the ‘Jell-O-like’ material surrounding it. This modification leads to the silencing of the X chromosome, effectively turning off the genes located on it. Understanding Xist’s mechanism is critical for developing gene therapies for X-linked disorders.
Can X chromosome inactivation lead to therapies for conditions like Fragile X Syndrome?
Yes, research into X chromosome inactivation has opened pathways for therapies specifically targeting conditions like Fragile X Syndrome. By reactivating the healthy gene that is usually inactivated, there is potential to correct the underlying genetic mutations causing these disorders. Jeannie Lee’s lab is developing methods aimed at achieving this goal.
Why is it challenging to treat neurodevelopmental disorders like Rett Syndrome linked to the X chromosome?
Treating neurodevelopmental disorders like Rett Syndrome is challenging because they are caused by mutations on X-linked genes, which are often inactivated in females. The complexities of X chromosome inactivation complicate the development of effective gene therapies. However, advances in understanding this process may lead to breakthroughs in restoring function to silenced genes.
What are the potential implications of reactivating inactivated X chromosomes for genetic therapies?
Reactivating inactivated X chromosomes has significant therapeutic implications for genetic disorders. It could enable access to functional copies of genes that are mutated, potentially curing conditions like Fragile X Syndrome while minimally impacting healthy genes. This offers a promising avenue for developing safe and effective treatments for X-linked genetic disorders.
How might male patients benefit from research on X chromosome inactivation even though they have only one X chromosome?
Although males have only one X chromosome and do not undergo traditional X chromosome inactivation, they could benefit from research on this topic. The mechanisms discovered may help in silencing specific harmful mutations present on their single X chromosome, thus providing a framework for targeted gene therapies for conditions like Fragile X Syndrome.
What future directions does Jeannie Lee envision for her research on X chromosome inactivation and genetic therapies?
Jeannie Lee envisions further refinement of methods to reactivate inactivated X chromosomes, along with conducting safety studies to assess the practicality of these therapies. With positive outcomes, her research could advance to clinical trials, paving the way for new treatment options for X-linked genetic disorders such as Fragile X Syndrome and Rett Syndrome.
| Key Points |
|---|
| Females have two X chromosomes while males have one, necessitating X chromosome inactivation in females. |
| Research led by Jeannie T. Lee focuses on the mechanisms of X chromosome inactivation, which presents a challenge for human cells. |
| X chromosome inactivation involves a jelly-like substance surrounding chromosomes that helps prevent tangling and allows for gene regulation. |
| A significant gene on the X chromosome produces Xist RNA, which modifies the surrounding jelly-like substance, enabling X-inactivation. |
| The Lee lab’s research could lead to therapies for genetic disorders like Fragile X Syndrome and Rett Syndrome by potentially reactivating inactivated X chromosomes. |
| Understanding X-inactivation offers hope for future treatments that might cure certain genetic conditions with minimal side effects. |
Summary
X chromosome inactivation is a vital biological process that ensures females, who possess two X chromosomes, do not express genes from both copies simultaneously. Research spearheaded by Jeannie T. Lee has unraveled the complexities of this mechanism, revealing the potential for future therapies targeting genetic disorders linked to the X chromosome. By focusing on the role of Xist RNA and the jelly-like substance surrounding chromosomes, scientists hope to develop methods to reactivate inactivated X chromosomes, restoring the function of healthy genes while mitigating the impact of mutations. This exciting advancement signifies a promising step towards treating conditions such as Fragile X Syndrome and Rett Syndrome.